TM 11-6140-203-14-1
contact with copper, which could occur if the nickel-
plating is scratched, corrosion will set in. For these
reasons, the battery must be carefully cleaned and
kept free of potassium carbonate deposits.
f. The potassium carbonate deposits, when dry, may
be removed with a nylon brush. Be sure that the filler
caps are secured before brushing. Any foreign matter
can contaminate the battery. If necessary, the tops of
the batteries may be flushed with water. Be sure that
the filler caps are tightly closed before flushing with
water. Batteries must be thoroughly dry before use.
g. Do not attempt to determine the state of charge
of a nickel-cadmium battery by a voltage check or by a
specific gravity check of the electrolyte. The electro-
lyte is not Changed by the chemical reaction which
takes place in the battery; the specific gravity is the
same whether the battery is charged or discharged. An
EL4GRO07
indication of the approximate state of charge is the
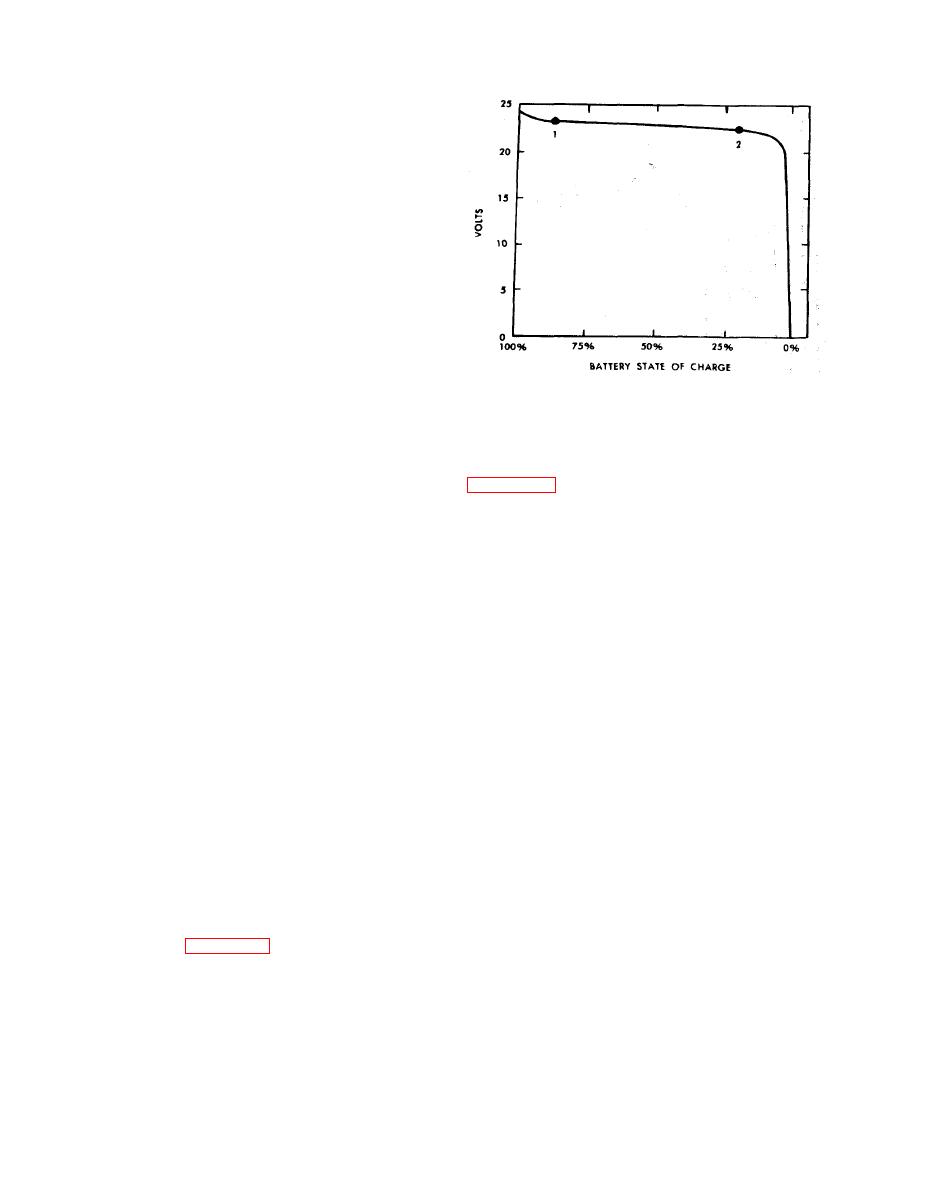
Figure 2-11. Typical Discharge Voltage Curve Under Moderate
amount of current the battery draws when it is con-
Load for Nickel-C!admium Battery.
nected to a constant potential charger. The higher the
state of charge, the less current the battery will draw.
2-20. Periodic Maintenance Cycling
should be performed at direct support or general sup-
The actual state of charge of a nickel-cadmium battery
port categories. For aircraft nickel-cadmium batteries,
is difficult to determine. It cannot be determined by
maintenance shown in flow chart is required every 100
the specific gravity of the electrolyte since it does not
flight hours or every 120 days (whichever shall occur
change during battery charge and discharge. The
first). For nonaircraft nickel-cadmium batteries, main-
methods described in a and b below may be used for a
tenance shown in flow chart is required quarterly or
general determination of charge condition. However
every 100 cycles (whichever shall occur first). The pe-
the most accurate method is to fully recharge the bat-
riodic maintenance cycle should result in the rebalanc-
tery before use if it has been charged and is on standby
ing of all cells in the battery, the reactivation of in-
for any length of time.
active plate material, and the replacement of defective
a. Battery Current Drawn From Constant Potential
cells. In addition, the battery as returned to the user
Source. A common method of checking the state of
contains the proper electrolyte level and a full charge.
charge is to connect the battery across a constant po-
tential charging source and observe the charging cur-
rent drawn by the battery as indicated by an ammeter
in series with the battery. The charging-source voltage
The proper height and method for adjusting the elec-
would normally be 1.5 volt times the number of cells;
trolyte level has caused considerable confusion in the
for example, 28.5 volts for a 19-cell, 24-volt battery at
past. This has been because of the wide variety of
70F. If the current drops to 1 or 2 amperes or less
charging methods, conditions of use, and available
within 5 minutes, the battery can be assumed to be in a
head space within each size and type of nickel-cad-
near state of full charge.
mium cell. Refer to TM 11-6140-203-14-2 or TM
b. Voltmeter Reading With Battery Under Load.
11-6140-203-14-3 for detailed procedures covering
Since the discharge voltage of a nickel-cadmium bat-
the battery being serviced.
tery is essentially constant until the battery is approx-
imately 90 percent discharged, voltmeter readings
a. Checking Electrolyte Level. Because a nickel-cad-
across the terminals while the battery is under load are
mium cell contains large amounts of electrolyte below
not too reliable. Figure 2-11 shows a typical voltage
the top of the plates, the fact that electrolyte is not
discharge curve under moderate load, and illustrates
visible in the partially discharged state is not neces-
the problem associated with voltmeter readings. A
sarily cause for alarm. When a battery has been
reading of approximately 24 volts may mean that the
charged on an aircraft bus, the electrolyte should be
battery is almost fully charged (point 1 on the curve)
visible above the cell plates immediately after the
or almost totally discharged (point 2 on curve).
charge is ended. If not, the electrolyte level in the cell


