TM 9-6140-200-14
1-10 When the Battery Charges:
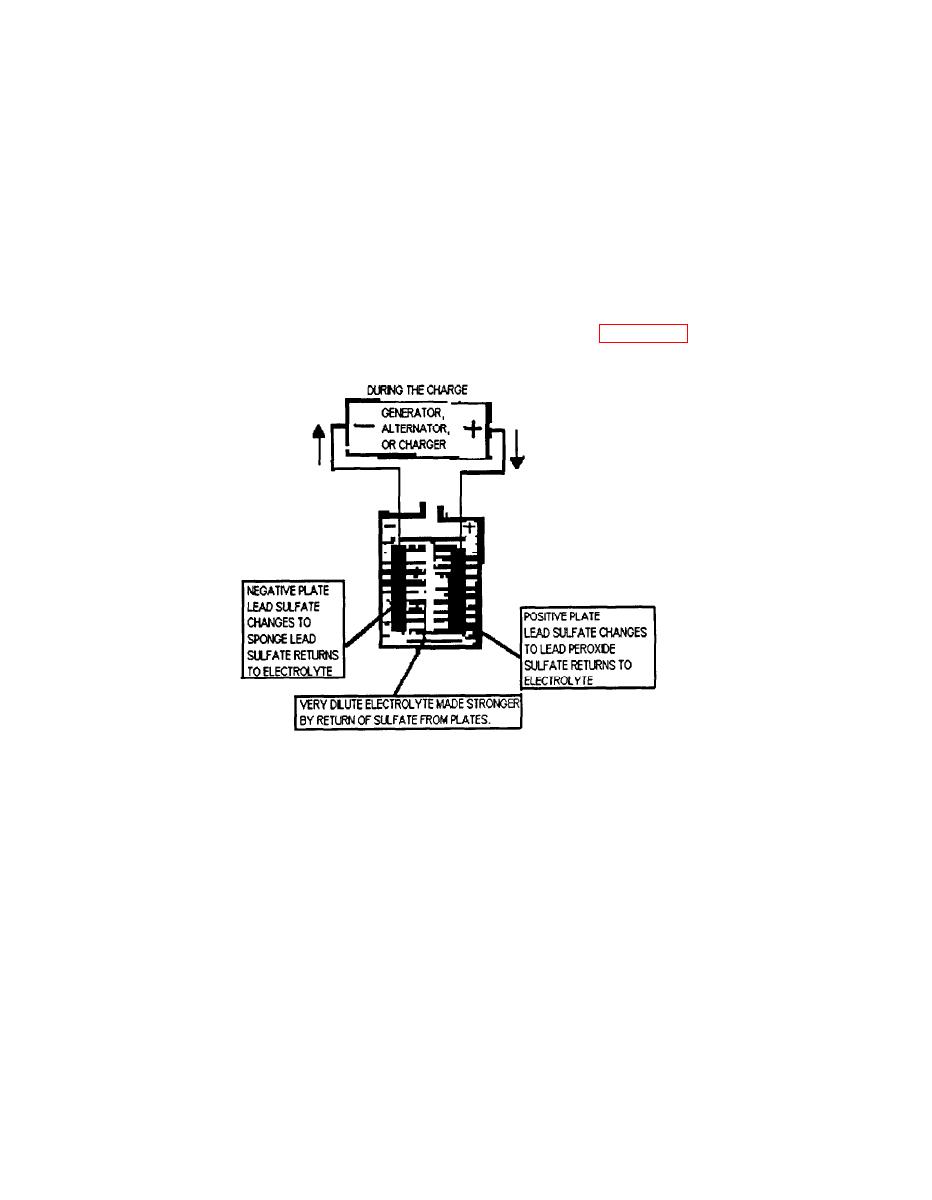
When an electrical current is passed through a lead-acid battery in a direction opposite
of the discharge, the lead sulfate is decomposed or broken up. The sulfate is expelled
or forced from positive or negative plates and returned to electrolyte. This restores the
electrolyte to its original strength before the battery discharge. The lead in the negative
plates and the lead peroxide in the positive plates are returned to the original condition.
The battery is ready to deliver electrical energy again. (see Figure 1-3)
Figure 1-3
A battery can produce gas when it is being charged. Hydrogen is given off at the
negative plate and oxygen at the positive. These gases result from the decomposition
of water. When battery gases it uses up water because it is being charged at a higher
rate than it can accept. This may be due to the fact that the battery is fully charged, its
plates are sulfated or it is too discharged to accept a charge. Generally, a battery will
gas near the end of a charge because the charge rate is too high for the battery to
accept it. Most automatic charger reduces the charge rate as the battery approaches
the fully charged state eliminating most of this gassing. Causing them to gas means
they are using water, which in sealed batteries, cannot be replaced.
DO NOT overcharge these battery.
1-7


